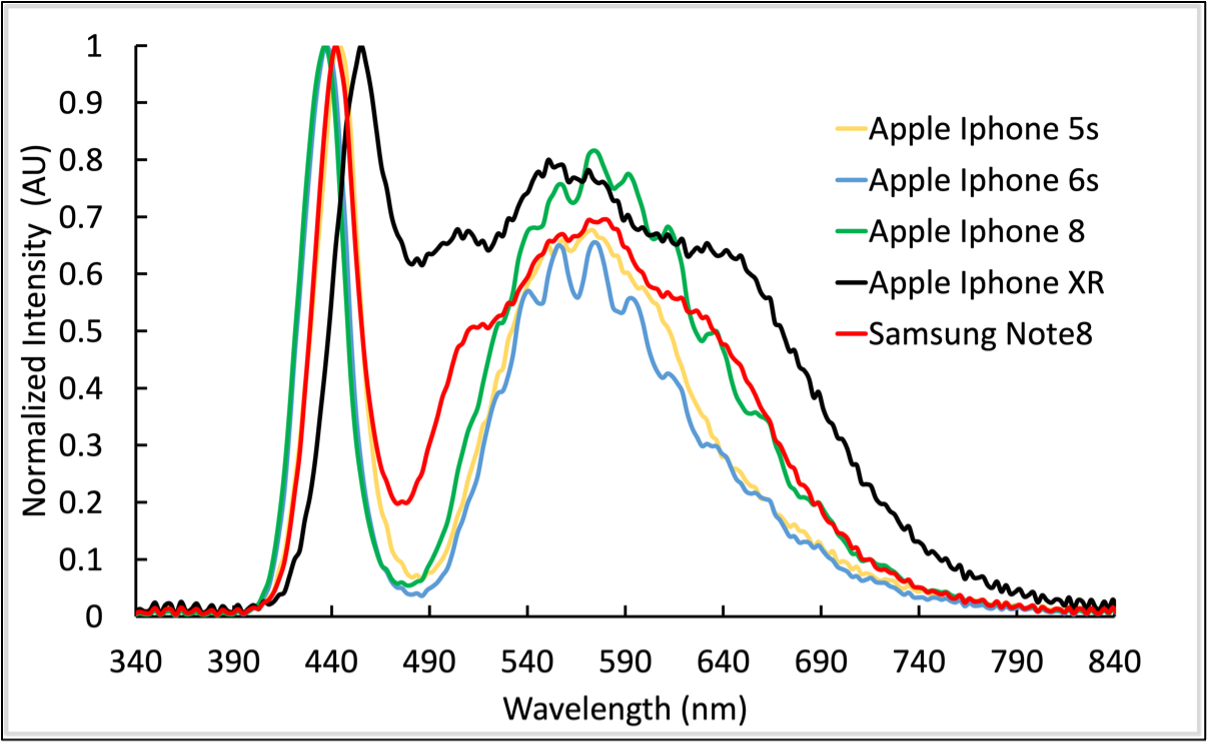
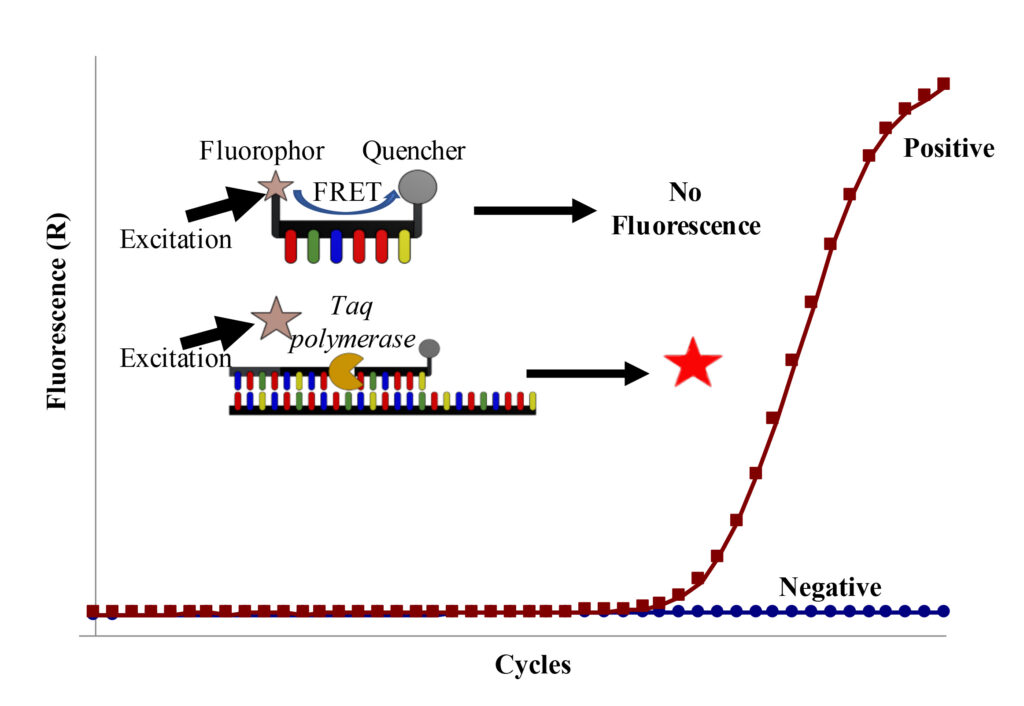
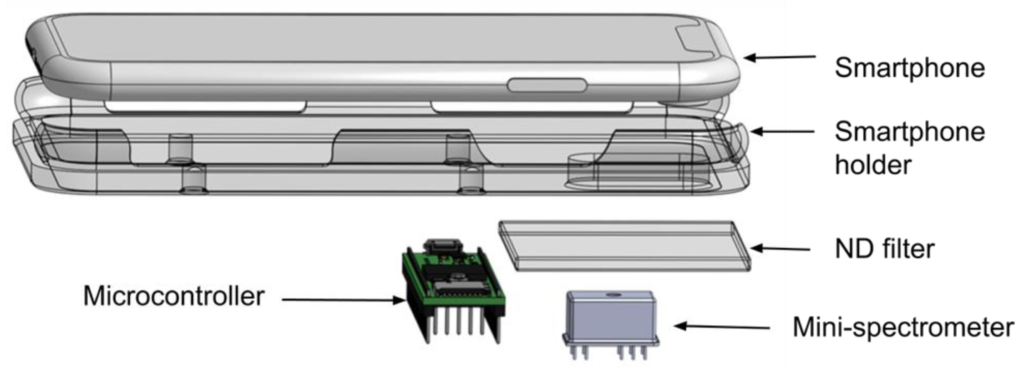
RESEARCH INTERESTS
Richard Willson’s Laboratory works on applications of biomolecular recognition to bioseparations, molecular diagnostics and process analytical technology.
We collaborate productively with groups in a broad range of disciplines. Over time, our group members have had expertise in chemical, biochemical and biomedical engineering, biochemistry, chemistry, chemical biology, computer science, materials science, app development, molecular genetics, microbiology, and nanofabrication.
BIOSEPARATIONS
(click images below to enlarge)
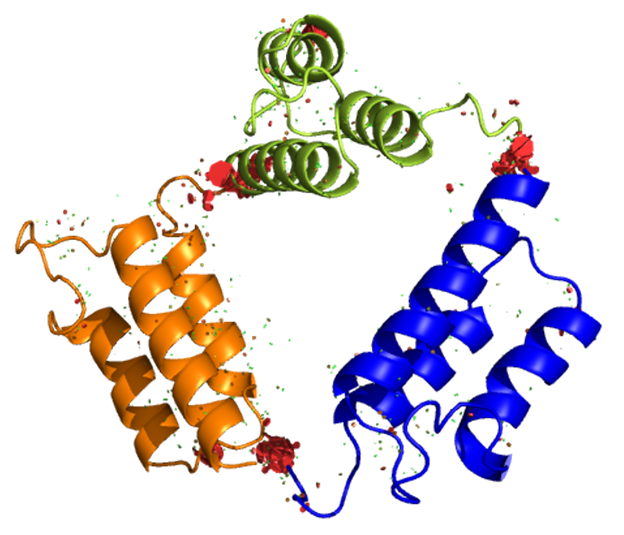
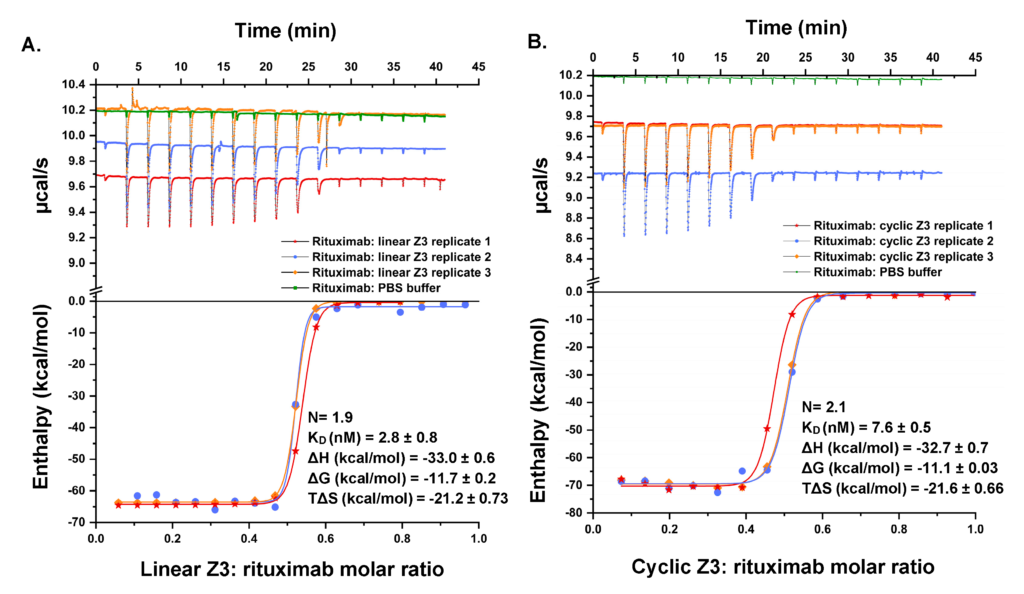
We have a long history of fundamental investigations of protein chromatography, including the first use of directed mutagenesis to rearrange surface charges to investigate selectivity, the first computational electrostatic modeling of protein/ion-exchanger interactions, the first use of titration microcalorimetry to characterize the energetics of protein ion-exchange adsorption, and demonstration of the role of stochastic clustering in protein adsorption and the superiority of clustered adsorbents. We and photophysicist C. Landes introduced (in PNAS) single-molecule methods to the study of protein chromatography; recent work focuses on antibody purification.
We are one of the few engineering groups developing novel methods of nucleic acid separations. These have been applied both at laboratory and production scale, including compaction agents and metal affinity capture of single-stranded nucleic acids. In recognition of his expertise in nucleic acid purification, Dr. Willson served on the Technical Advisory Board of Moderna, Inc. which has an mRNA-based vaccine for COVID-19 in advanced trials. One of his former students in this area is Head, Nucleic Acid Process Development at Moderna.
IMMUNOCHROMATOGRAPHIC DIAGNOSTICS
(click images below to enlarge)
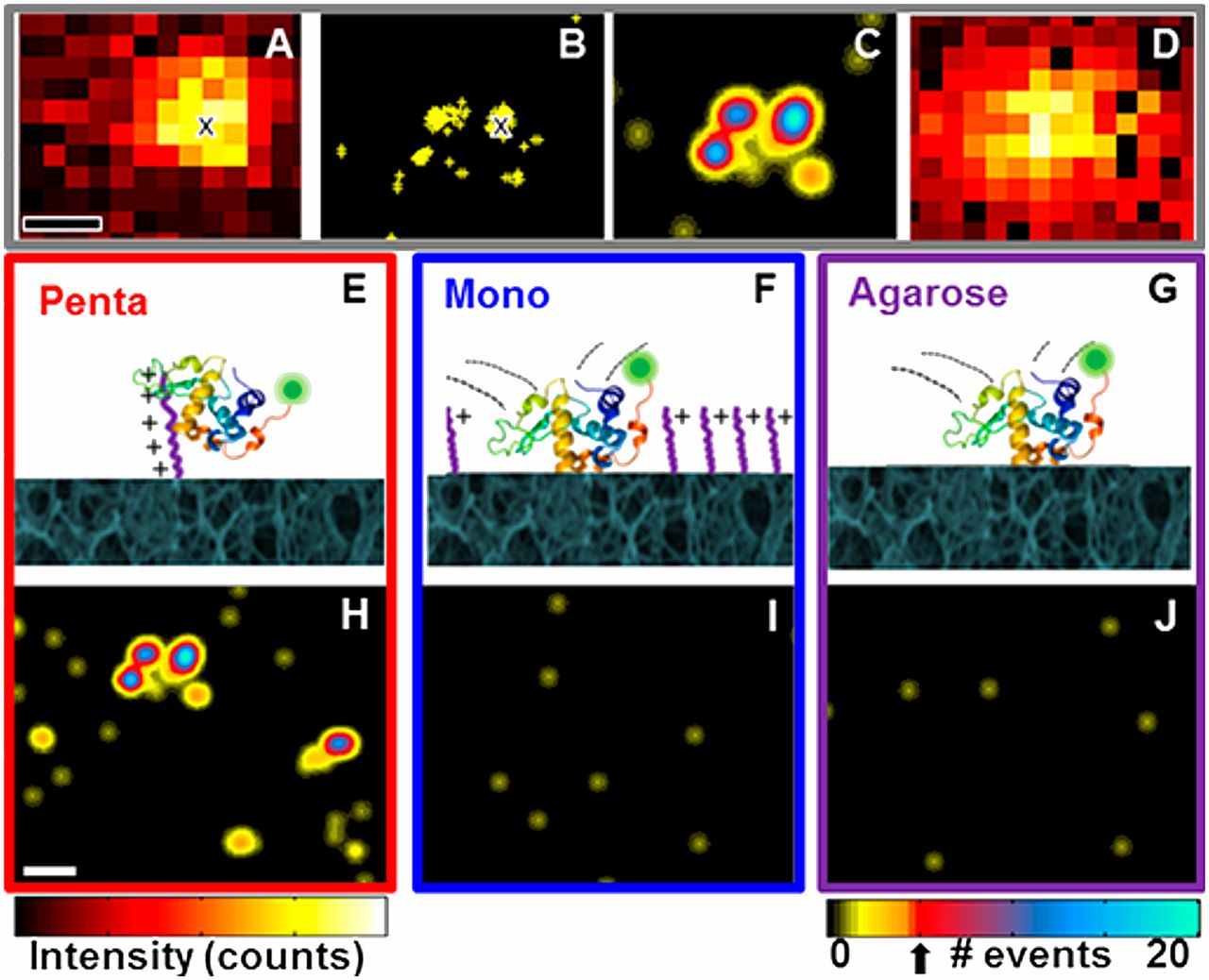
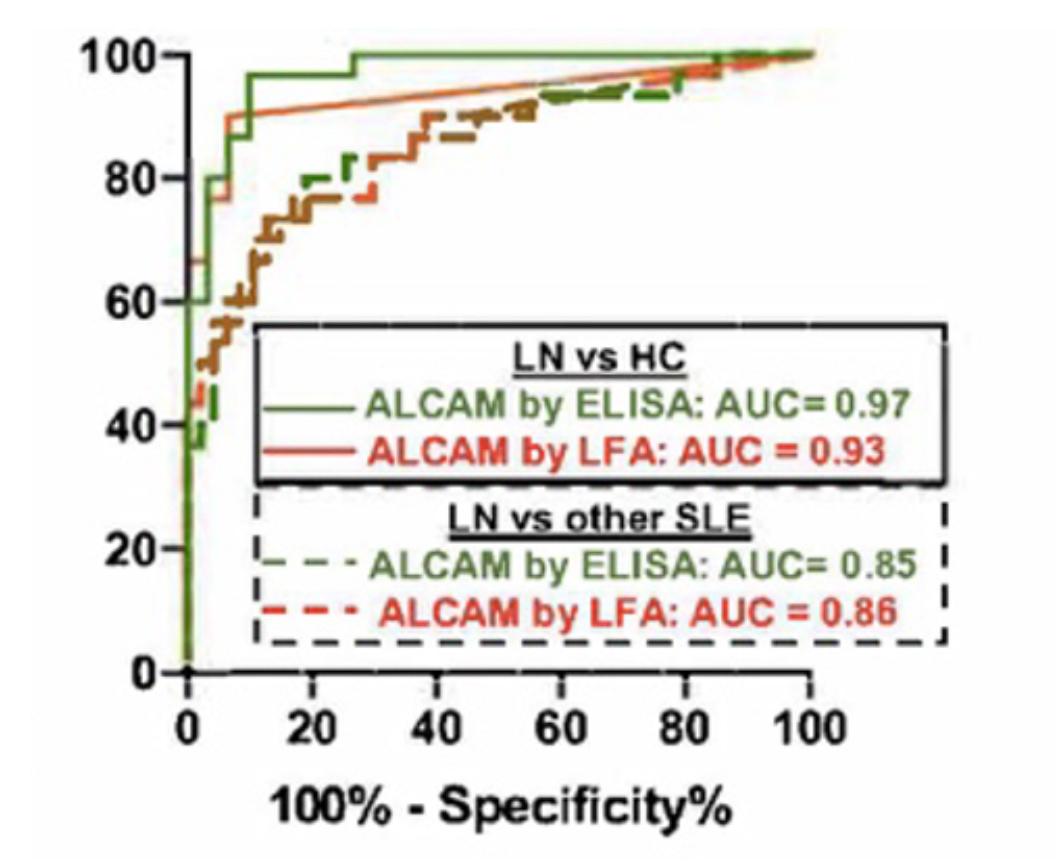
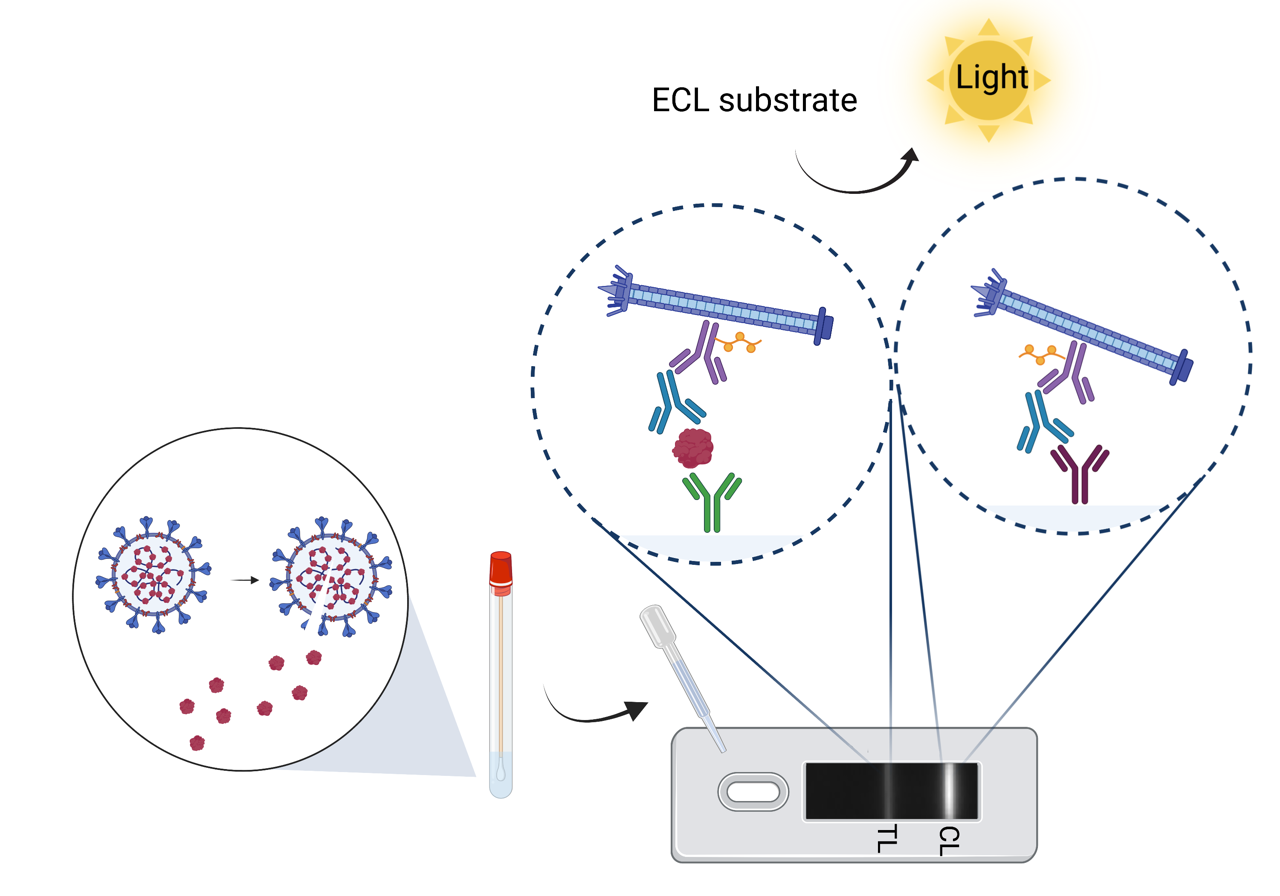
Recently, we have combined our experience in chromatography and affinity technologies to develop improved immuno-chromatographic assays. This diagnostic format, best known as the basis of the home pregnancy test, relies on the capture of flowing reporter particles decorated with antibodies on lines of capture antibodies when bridged by target analyte. We first replaced the conventional gold and polystyrene reporter particles by engineered filamentous phage particles, which are neutrally buoyant, have high surface area, and are under Darwinian selection for low non-specific binding and aggregation. Phage lateral-flow tests can be up to 1000 times as sensitive as standard assays, opening new applications for this convenient assay format, including PAT and the direct detection of viral infections in blood.
We also introduced strontium aluminate phosphorescent nanoparticles derived from cheap glow-in-the-dark materials as lateral flow reporters, and demonstrated their superior performance in sensitive, quantitative lateral flow immunoassays. This technology was spun off by group members as Luminostics, Inc. which was accepted into Y Combinator, the outstanding incubator of Airbnb, Stripe and Dropbox. Luminostics is now VC-funded and pursuing human clinical trials of a home COVID-19 diagnostic with Sanofi
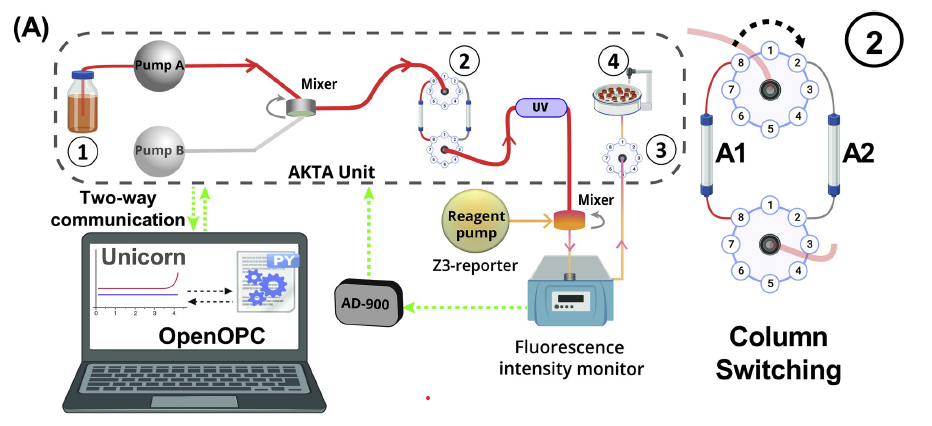
PROCESS ANALYTICAL TECHNOLOGY
Recently, we’ve applied our analytical methods to Process Analytical Technology (PAT), monitoring downstream processes to maximize efficiency and product quality. The lab recently constructed Protein A-based mix-and-read fluorescence reporters for antibody Fc fragments. These reporters should be quite useful for clone selection and screening, and routine measurements, and we also demonstrated their use in continuous monitoring of mAb breakthrough for Protein A capture column process control.